The news of the Novo Nordisk (Novo Holdings) intent to acquire Catalent has taken the…
How to Implement Continued Process Verification (CPV) for Process Monitoring
ICH Q10 Guidance and Process Validation Guidance published by FDA in 2011 emphasizes both routine process monitoring and Continued Process Verification (CPV) as a key aspect of the third and final stage of process validation and its life-cycle management
“Pharmaceutical Companies should plan and execute a system for the monitoring of process performance and product quality to ensure a state of control is maintained. An effective monitoring system provides assurance of the continued capability of processes and controls to meet product quality and to identify areas for continual improvement” – ICH Q10 Guidance
“We recommend continued monitoring and sampling of process parameters and quality attributes at the level established during the process qualification stage until sufficient data are available to generate significant variability estimates…Process variability should be periodically assessed and monitoring adjusted accordingly” – FDA Process Validation Guidance 2011
Unsurprisingly. we detected an update in industry interest in CPV after the above guidance was released. This is a good thing, but it’s unfortunate that it takes regulatory pressure to move pharmaceutical companies towards best practices for capturing and managing manufacturing data that potato chip and soap manufacturers adopted many, many years ago. In the late nineteenth century, Guinness, the beer brewer, was the first company to adopt statistical process control (SPC) for an industrial process, yet engineers and scientists struggle to get their hands on process and manufacturing data at many pharmaceutical companies in the 21st century. I often said earlier in my career my biggest problem was getting my hands on the data I needed. Once I had it, I was invariably able to solve the problem or improve the process.
A well done and detailed case study has been published by BPOG (Biophorum Operations Group) on how to implement CPV. Though this case study is very detailed, here below is summarized in a flow chart the steps involved in setting up CPV. This gives an idea of what procedures, tools and efforts needed to rapidly set up a CPV program at your organization.
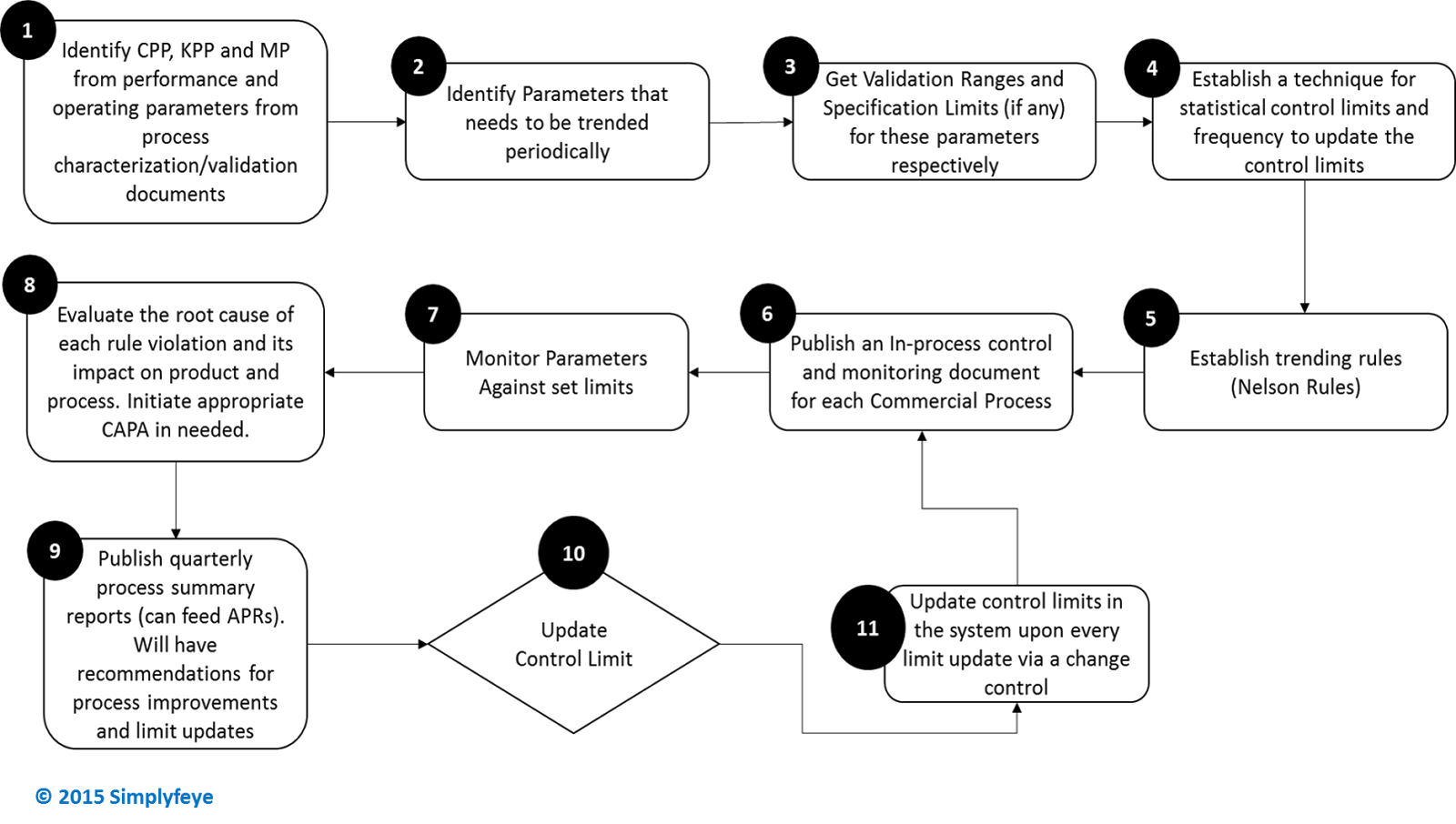
Steps 1 through 4 involve scientific judgment and hence cannot be automated directly. However, use of a CPV tool during these stages can greatly help in identifying key and critical parameters by easing analysis and providing new insights.
Without a CPV solution, Steps 5 through 11 are extremely labor intensive and it can be a daunting task to routinely monitor hundreds of parameters, investigate trends, and update/adjust control limits while trying to monitor process variability and capability. If your site/organization is a multi-product commercial manufacturing site, this activity becomes nearly impossible to perform manually and to keep up with the frequent reviews of processes while living with limited resources (FTE’s). Most of the steps from Steps 5 through 11 can easily be automated with a CPV software solution with capabilities for:
- trending and SPC (statistical process control)
- data integration and warehousing of all relevant historical process information
- lot traceability and genealogy
- performing correlations across process steps for quick troubleshooting
- overlaying batch trends
- templating specific process parameters for routine periodic trending
- historical limits management
- validated reporting with both standing and ad-hoc reporting, including Annual Product Review reports.
Few tools in this space have all these capabilities. Not too long ago, organizations needed to develop these tools in-house. Now, very economical COTS (commercial off the shelf) solutions exist, exist. Cloud hosting options can also enable easy implementation. Request more information



This Post Has 0 Comments